Chemical reactions-
The transformation of chemical substance into a new chemical substance by making and breaking of bonds between different atoms is known as Chemical Reaction.
Signs of a chemical reaction
These factors denote that a chemical reaction has taken place- change of state of substance, change of color of substance,evolution of heat, absorption of heat, evolution of gas and evolution of light.

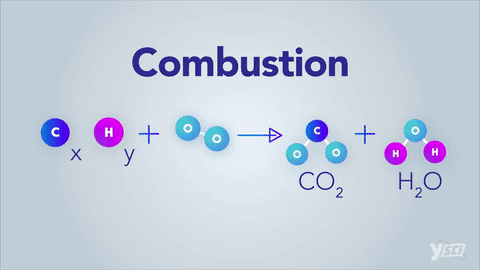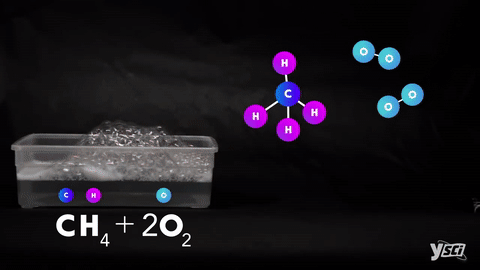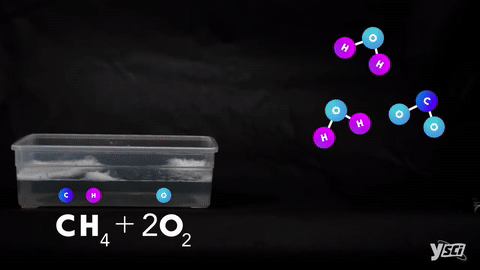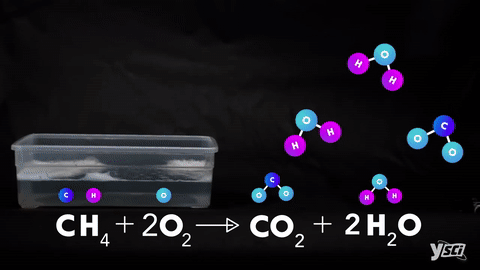
Chemical Equation:
The representation of chemical reaction by means of symbols of substances in the form of formulae is called chemical equation.
E.g. - H2 + O2 ⇒ H2O

Balanced Chemical Equation:
A balanced chemical equation has number atoms of each element equal on both left and right sides of the reaction.

*Note- According to Law of Conservation of Mass, mass can neither be created nor destroyed in a chemical reaction. To obey this law, the total mass of elements present in reactants must be equal to the total mass of elements present in products.
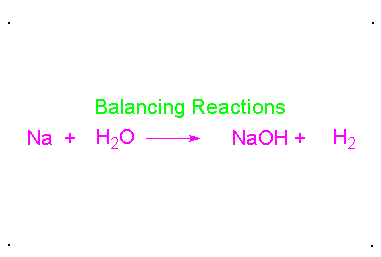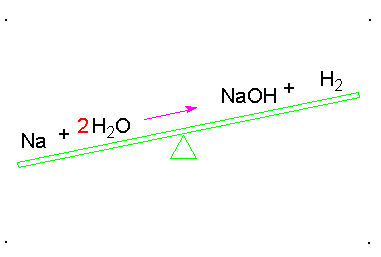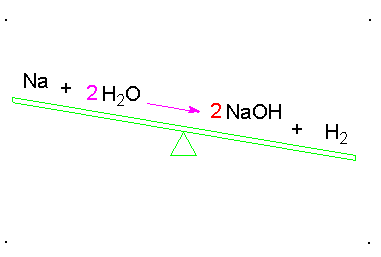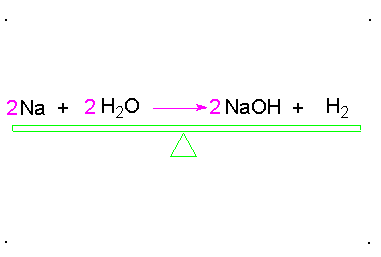
Types of Chemical Reactions-

I. Combination- When two elements or one element and one compound or two compounds combines to give one single product.
II. Decomposition- Splitting of a compound into two or more simple products.

III. Displacement- It takes place when a more reactive metal displaces a less reactive metal.

IV. Double displacement- Reactions in which ions are exchanged between two reactants forming new compounds are called double displacement reactions.

V. Precipitation- The insoluble compound called precipitate forms in this reaction.

VI. Exothermic- Reactions which produce energy are called exothermic reaction. Most of the decomposition reactions are exothermic.

VII. Endothermic- Reactions which absorb energy are called endothermic reaction. Most of the combination reactions are endothermic.

VIII. Oxidation: Gain of oxygen or removal of hydrogen or metallic element from a compound is known as oxidation
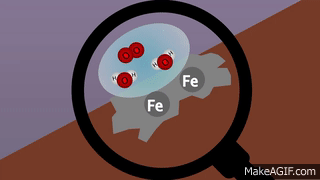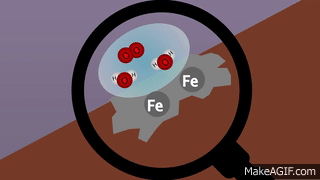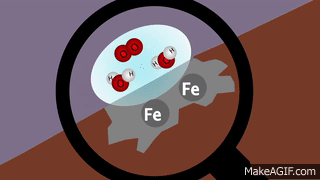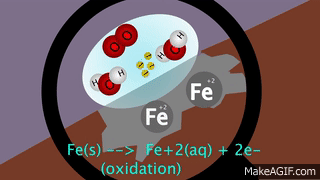 .
.IX. Reduction: Addition of hydrogen or removal of oxygen from a compound is called reduction.
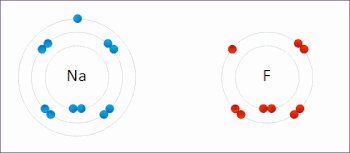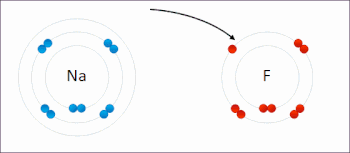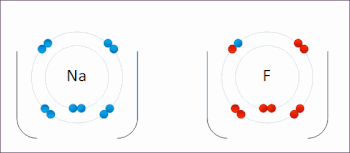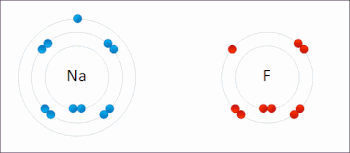
X. Redox- A chemical reactions where oxidation and reduction both take place simultaneously are also known as redox reaction.
Eg - NaOH + HCl ⇒ NaCl + H2O

6. Rusting- When iron reacts with oxygen and moisture forms a red substance called rust.


7. Rancidity- Oils and fats when get oxidized on exposure to air show a change in taste and smell.
8. Corrosion- Metals when attacked by oxygen, water, acids, gases, present in air changes its surface which is called corrosion.



 |
nice concept
ReplyDelete